My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Without gluten
Cardamom (ELETTARIA CARDAMOMOMUM) whole nut.
Cardamom (ELETTARIA CARDAMOMUM) whole nut
Cardamom is a spice that is widely used in the cuisine of India and Middle Eastern countries in the preparation of various dishes such as curries, rice, desserts, cakes and cookies among others. It is also used to flavor drinks such as tea and coffee in Arab countries
In Scandinavian countries it is often used as an ingredient in pastries and spiced breads, accompanied by cloves, cinnamon and ginger
It brings to your Gin tonic its carminative, stimulant, antispasmodic, sialagogue, orexigenic and aromatic properties. We recommend 4 grains per glass. Between sweet, spicy and citrus, its flavor goes well with any gin.
Gin and tonic
ORGANOLEPTIC CHARACTERISTICS: Appearance: oval to oblong-shaped capsules, with a rounded part with three angles Color: Shades of light green to brown or cream and white Color: Shades of light green to brown or cream and white
Odor: Characteristic. Absence of foreign odors Flavor: Characteristic. Sweet and very fresh Nutritional value (in 100 g. Bibliographic data): Flavor: Characteristic
Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. Store in a cool, dry place, away from strong odors and sources of contamination
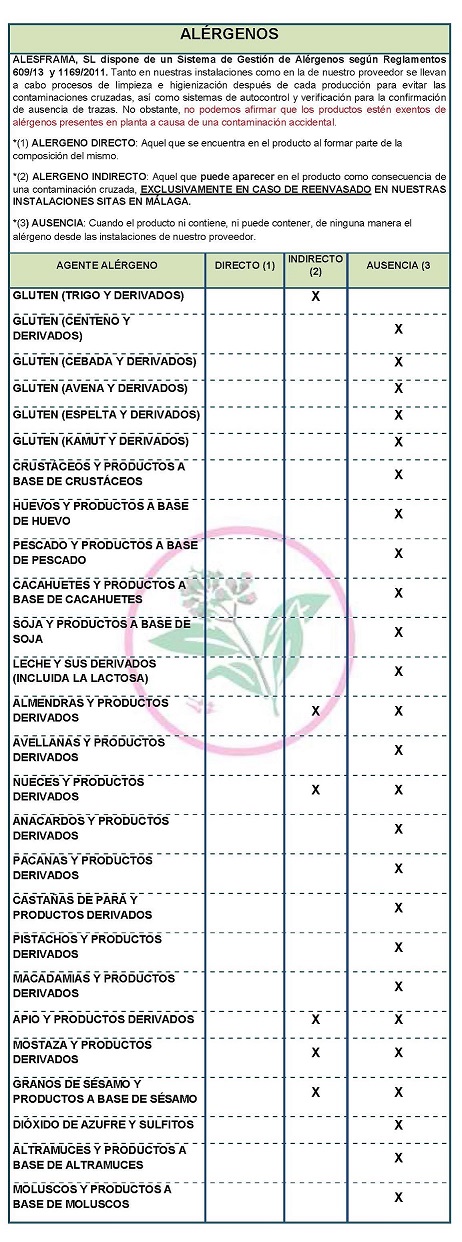
DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the technical data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free from allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLING DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to ROYAL DECREE 1334/1999, of July 31, which approves the General Standard for labeling, presentation and advertising of foodstuffs and its subsequent amendments. And Annex II of REGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 October 2011 on the provision of food information to consumers and amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC, and 2008/5/EC, and Commission Regulation (EC) No 608/2004. The product does not fall within the scope of Regulations (EC) 1829/2003 (GMO food and feed) and (EC) 1830/2003. That is, it does not contain or consist of GMOs, is not produced from GMOs and does not contain ingredients produced from GMOs. Therefore, it is not subject to the specific labeling requirements set forth in the aforementioned regulation. NON-GENETICALLY MODIFIED STATEMENT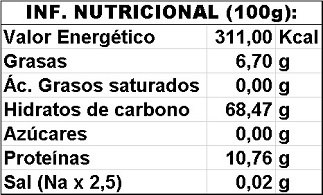
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
Cardamom (ELETTARIA CARDAMOMOMUM) whole nut.
Cardamom (ELETTARIA CARDAMOMUM) whole nut
Cardamom is a spice that is widely used in the cuisine of India and Middle Eastern countries in the preparation of various dishes such as curries, rice, desserts, cakes and cookies among others. It is also used to flavor drinks such as tea and coffee in Arab countries
In Scandinavian countries it is often used as an ingredient in pastries and spiced breads, accompanied by cloves, cinnamon and ginger
It brings to your Gin tonic its carminative, stimulant, antispasmodic, sialagogue, orexigenic and aromatic properties. We recommend 4 grains per glass. Between sweet, spicy and citrus, its flavor goes well with any gin.
Gin and tonic
ORGANOLEPTIC CHARACTERISTICS: Appearance: oval to oblong-shaped capsules, with a rounded part with three angles Color: Shades of light green to brown or cream and white Color: Shades of light green to brown or cream and white
Odor: Characteristic. Absence of foreign odors Flavor: Characteristic. Sweet and very fresh Nutritional value (in 100 g. Bibliographic data): Flavor: Characteristic
Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. Store in a cool, dry place, away from strong odors and sources of contamination
DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the technical data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free from allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLING DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to ROYAL DECREE 1334/1999, of July 31, which approves the General Standard for labeling, presentation and advertising of foodstuffs and its subsequent amendments. And Annex II of REGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 October 2011 on the provision of food information to consumers and amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC, and 2008/5/EC, and Commission Regulation (EC) No 608/2004. The product does not fall within the scope of Regulations (EC) 1829/2003 (GMO food and feed) and (EC) 1830/2003. That is, it does not contain or consist of GMOs, is not produced from GMOs and does not contain ingredients produced from GMOs. Therefore, it is not subject to the specific labeling requirements set forth in the aforementioned regulation. NON-GENETICALLY MODIFIED STATEMENT

APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
