My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Without gluten
Milled fruits of Capsicum frutencens Linnaeus. Its color is dark to bright red. Characteristic odor and strongly pungent flavor
Product obtained by grinding the dried, healthy and clean fruits of the varieties of Capsicum frutescens and/or Capsicum Annuum
Its color is dark to bright red. Characteristic odor and strongly pungent flavor
It has a strong pungent flavor
Milled cayenne is the powder resulting from grinding the fruits of one or more species of Capsicum (chili or chili bell pepper), previously dried.
Origin: India/China/Spain/Peru
Capsicum
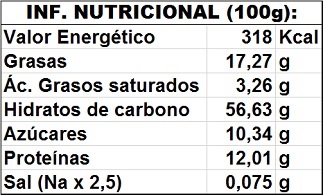
Nutritional value calculated in g/100g:
DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
NOT contain GLUTEN.
ALLERGENS: Does not contain allergens as ingredients.
Allergens: Does not contain allergens as ingredients
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLING
In accordance with Directive 2003/89/EC the following is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances may cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 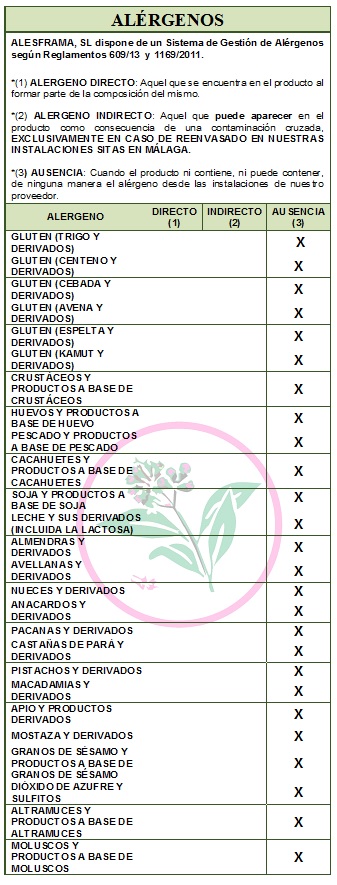
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
SUITABLE FOR CELIACS : YES
Other non-culinary uses:
Milled fruits of Capsicum frutencens Linnaeus. Its color is dark to bright red. Characteristic odor and strongly pungent flavor
Product obtained by grinding the dried, healthy and clean fruits of the varieties of Capsicum frutescens and/or Capsicum Annuum
Its color is dark to bright red. Characteristic odor and strongly pungent flavor
It has a strong pungent flavor
Milled cayenne is the powder resulting from grinding the fruits of one or more species of Capsicum (chili or chili bell pepper), previously dried.
Origin: India/China/Spain/Peru
Capsicum
Nutritional value calculated in g/100g:

DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
NOT contain GLUTEN.
ALLERGENS: Does not contain allergens as ingredients.
Allergens: Does not contain allergens as ingredients
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLING
In accordance with Directive 2003/89/EC the following is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances may cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
SUITABLE FOR CELIACS : YES
Other non-culinary uses:
