My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Without gluten
Floral lavender (LAVENDER) whole flower (LAVANDULA SPICA L.) leaves and tops healthy, clean and dried.
Among its properties are antispasmodic and antibacterial qualities. It is an excellent antiseptic that is recommended for skin infections and skin wounds. Its consumption helps in problems of digestive spasms.
It is an excellent antiseptic that is recommended for skin infections and skin wounds
The flowers of lavender have healing properties in throat affections, such as irritations; it is sedative and useful in neuralgias. The active parts have mainly external applications, in baths, compresses and disinfection of sores and wounds. It is useful in the first symptoms of flu-like processes. It is also stimulant, antispasmodic (mild sedative), good medicine against melancholy, nervous disorders and dizziness; also popular in digestive applications
The plant contains up to 3% of highly perfumed essential oil (oleum lavandulae), which encloses linalin acetate, geraniol and borneol, as well as 12% tannins. Lavender oil is used in the composition of anti-rheumatic preparations. The alcohol of lavender is very suitable for rubbing, to add to baths activating the circulation or relieving fatigue. The cosmetic industry is the largest consumer of lavender essence; it is one of the components of eau de cologne. The dried flowers are used to scent clothes and repel moths.
The lavender is an excellent melliferous plant, and one of the best known and most popular for its aroma and medicinal virtues. Its flavor is pungent, somewhat bitter.
It has a pungent, somewhat bitter taste
PROPERTIES: Diuretic, sudorific, cholagogue, stimulant, carminative, antispasmodic, stomachic, antiseptic. It is also used as an antiseptic
INDICATIONS:
Hypertension, meteorism, convulsive cough, whooping cough, oliguria, slow digestion. In external use
In external use: pharyngitis, stomatitis. In external use: pharyngitis, stomatitis
PRECAUTIONS: No described
Not described. Precautions
CONTRAINDICATIONS: Not described. CONTRAINDICATIONS:(SOURCE
(SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) (SOURCE
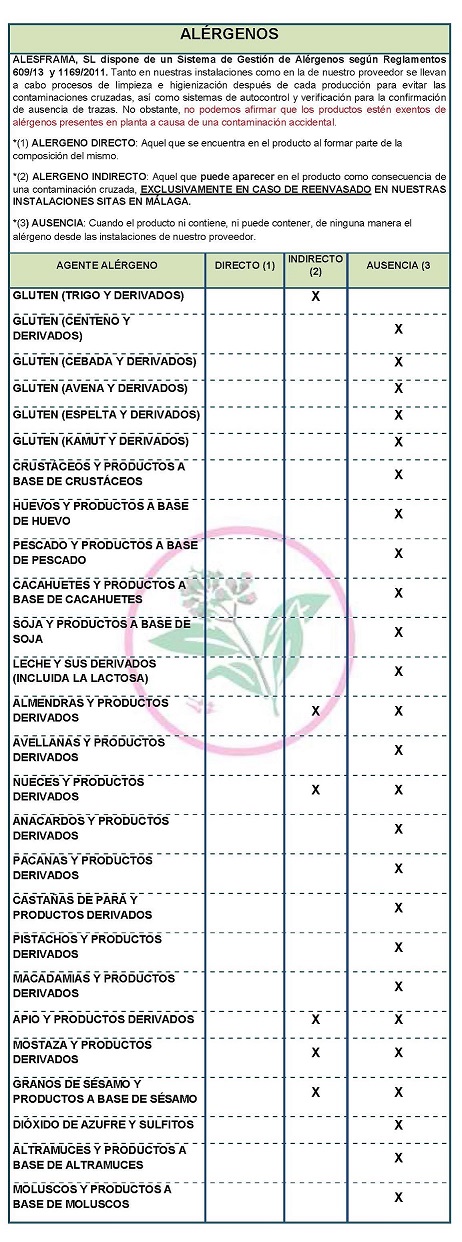
Use: Not described. Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS: HALAL FITNESS : YES, official certificate not available DIABETICS SUITABILITY : YES FIT FOR VEGETARIANS : YES FIT FOR VEGANISTS : YES FIT FOR VEGANS : YES CELIACS SUITABILITY : NO NOT SUITABLE FOR CELIACS : NO
Floral lavender (LAVENDER) whole flower (LAVANDULA SPICA L.) leaves and tops healthy, clean and dried.
Among its properties are antispasmodic and antibacterial qualities. It is an excellent antiseptic that is recommended for skin infections and skin wounds. Its consumption helps in problems of digestive spasms.
It is an excellent antiseptic that is recommended for skin infections and skin wounds
The flowers of lavender have healing properties in throat affections, such as irritations; it is sedative and useful in neuralgias. The active parts have mainly external applications, in baths, compresses and disinfection of sores and wounds. It is useful in the first symptoms of flu-like processes. It is also stimulant, antispasmodic (mild sedative), good medicine against melancholy, nervous disorders and dizziness; also popular in digestive applications
The plant contains up to 3% of highly perfumed essential oil (oleum lavandulae), which encloses linalin acetate, geraniol and borneol, as well as 12% tannins. Lavender oil is used in the composition of anti-rheumatic preparations. The alcohol of lavender is very suitable for rubbing, to add to baths activating the circulation or relieving fatigue. The cosmetic industry is the largest consumer of lavender essence; it is one of the components of eau de cologne. The dried flowers are used to scent clothes and repel moths.
The lavender is an excellent melliferous plant, and one of the best known and most popular for its aroma and medicinal virtues. Its flavor is pungent, somewhat bitter.
It has a pungent, somewhat bitter taste
PROPERTIES: Diuretic, sudorific, cholagogue, stimulant, carminative, antispasmodic, stomachic, antiseptic. It is also used as an antiseptic
INDICATIONS:
Hypertension, meteorism, convulsive cough, whooping cough, oliguria, slow digestion. In external use
In external use: pharyngitis, stomatitis. In external use: pharyngitis, stomatitis
PRECAUTIONS: No described
Not described. Precautions
CONTRAINDICATIONS: Not described. CONTRAINDICATIONS:(SOURCE
(SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) (SOURCE
Use: Not described. Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS: HALAL FITNESS : YES, official certificate not available DIABETICS SUITABILITY : YES FIT FOR VEGETARIANS : YES FIT FOR VEGANISTS : YES FIT FOR VEGANS : YES CELIACS SUITABILITY : NO NOT SUITABLE FOR CELIACS : NO

