My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Without gluten
Dried bark from branches of Salix alba (SAUCE WHITE)
Salix alba contains in its bark a compound called salicin, from which salicylic acid is obtained, the predecessor of aspirin
Salix alba contains in its bark a compound called salicin, from which salicylic acid, the predecessor of aspirin, is obtained.
Salix alba is the most common type of salicylic acid in the world
Healthy, clean and dried leaves of Medicago sativa
USE
Add a teaspoon of chopped alfalfa leaves and pour 200 ml of very hot water. Cover and let stand for 5 minutes. Then strain and drink little by little as it cools.
CULT
GROWING AND HARVESTING: PART PLANT: BARK PRESENTATION: CUTTINGS COUNTRY OF ORIGIN: POLAND COLLECTION: SIVESTRIAN ORGANOLEPTIC CHARACTERISTICS CARACTERISTICS COLOR: BROWN SPELL: CHARACTERISTIC APPEARANCE: CORRECT Preservation Keep in a cool, dry place, away from strong odors and sources of contamination
Salix alba contains in its bark a compound called salicin, from which salicylic acid, the predecessor of aspirin, is obtained. Salix alba has a salicin compound in its bark, from which salicylic acid, the predecessor of aspirin, is obtained
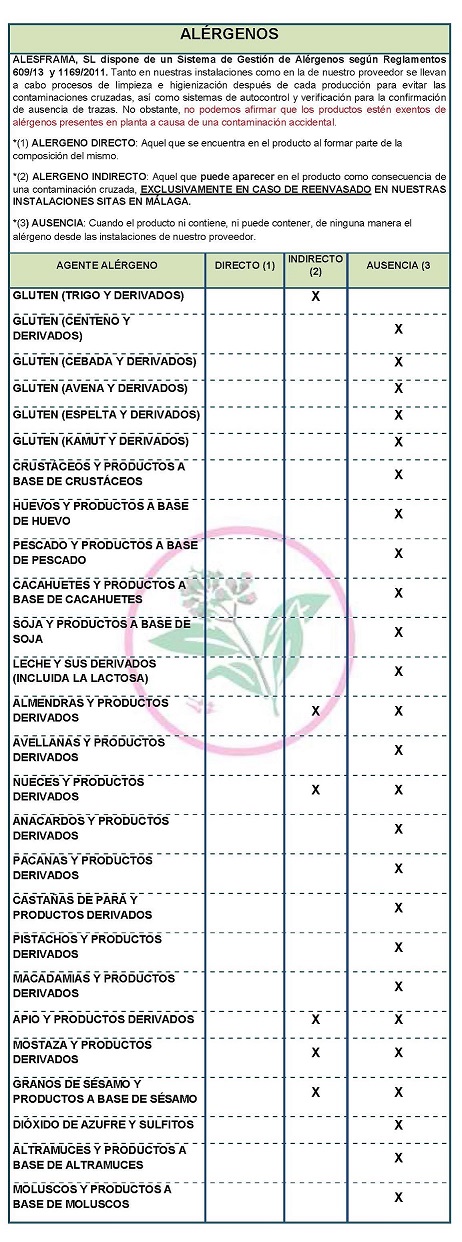
Salicin has analgesic, antipyretic and anti-inflammatory, anticoagulant, astringent and depurative properties, thus relieving pain symptoms and all other related symptoms. DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLINGProperties

In accordance with Directive 2003/89/EC the following is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances may cause allergies.
DECLARATION OF NOT GENETICALLY MODIFIED
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIAC SUITABILITY : NO
SUIT FOR CELIACS : NO
Dried bark from branches of Salix alba (SAUCE WHITE)
Salix alba contains in its bark a compound called salicin, from which salicylic acid is obtained, the predecessor of aspirin
Salix alba contains in its bark a compound called salicin, from which salicylic acid, the predecessor of aspirin, is obtained.
Salix alba is the most common type of salicylic acid in the world
Healthy, clean and dried leaves of Medicago sativa
USE
Add a teaspoon of chopped alfalfa leaves and pour 200 ml of very hot water. Cover and let stand for 5 minutes. Then strain and drink little by little as it cools.
CULT
GROWING AND HARVESTING: PART PLANT: BARK PRESENTATION: CUTTINGS COUNTRY OF ORIGIN: POLAND COLLECTION: SIVESTRIAN ORGANOLEPTIC CHARACTERISTICS CARACTERISTICS COLOR: BROWN SPELL: CHARACTERISTIC APPEARANCE: CORRECT Preservation Keep in a cool, dry place, away from strong odors and sources of contamination
Salix alba contains in its bark a compound called salicin, from which salicylic acid, the predecessor of aspirin, is obtained. Salix alba has a salicin compound in its bark, from which salicylic acid, the predecessor of aspirin, is obtained
Salicin has analgesic, antipyretic and anti-inflammatory, anticoagulant, astringent and depurative properties, thus relieving pain symptoms and all other related symptoms. DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND ALLERGEN LABELLINGProperties

In accordance with Directive 2003/89/EC the following is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances may cause allergies.

DECLARATION OF NOT GENETICALLY MODIFIED
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIAC SUITABILITY : NO
SUIT FOR CELIACS : NO
