My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Vegan

Without gluten

Vegan
Mugwort is one of the oldest medicinal plants in the world. According to Greek mythology, the goddess Artemisia, protector of nature, helped women in childbirth; hence the name of this plant that has, among others, properties against menstrual or postpartum pains.
Of potent bitter taste,itis known as Wormwood, bitter mugwort or holy herb.
Mugwort (ARTEMISIA VULGARIS) Cut plant.
Mugwort is one of the oldest medicinal plants in the world
Artemisia is one of the oldest medicinal plants in the world. According to Greek mythology, the goddess Artemisia, protector of nature, helped women in childbirth; hence the name of this plant that has, among others, properties against menstrual pain or postpartum.
It is a medicinal herb whose properties have been known since the time of the pharaohs. Of potent bitter taste because of one of its components, the absintin.
It is a medicinal herb whose properties have been known since the time of the pharaohs
PROPERTIES: Antimicrobial, emmenagogue, astringent, aperient and eupeptic. INDICATIONS:
Anorexia, slow digestion, amenorrhea, dysmenorrhea. PRECAUTIONS:
Do not use it continuously. Do not use it continuously
CONTRAINDICATIONS: CONTRAINDICATIONS: Pregnancy and pregnancy
Pregnancy and lactation. CONTRAINDICATIONS
(SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) (SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) Menstrual disorders : The infusion of the leaves at a rate of 10 g per liter gives benefits as an emmenagogue, so it is recommended in menstrual disorders, such as dysmenorrhea and amenorrhea. At the digestive level : Being a bitter tonic, it is recommended to improve bile secretion, as well as appetite stimulant. At the digestive level : As a bitter tonic, it is recommended to improve bile secretion, as well as appetite stimulant
For diabetes : Prolonged treatments based on mugwort, in a minimum of 3 months, are recommended for cases of diabetes, as it has certain qualities that lower glucose
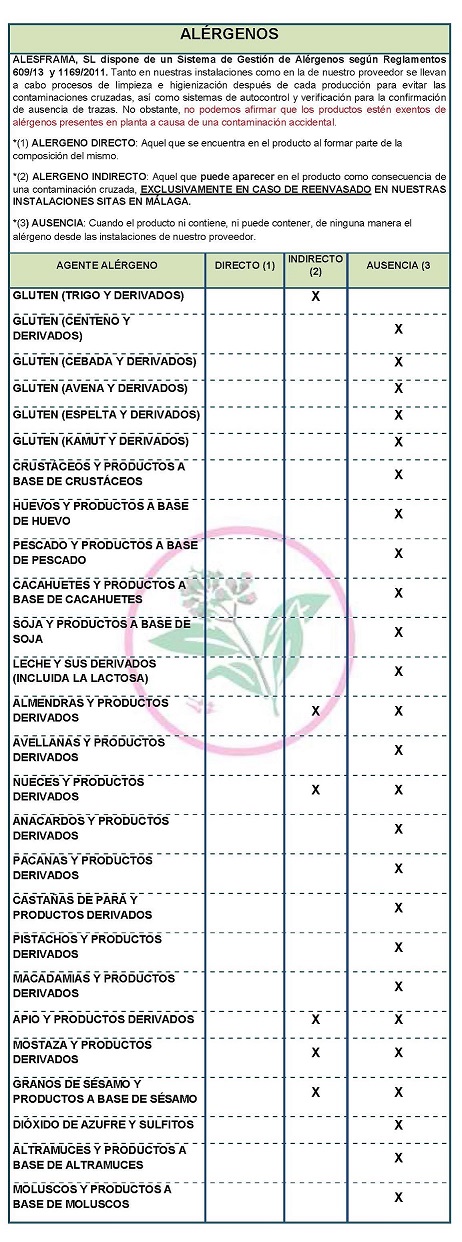
Organoleptic characteristics: Preservation Keep in a cool, dry place, away from strong odors and sources of contamination. DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED Benefits of Mugwort
In those cases where menstruation does not come and there is no likelihood of pregnancy is very useful to accelerate the process and end the wait by applying vaginal vapors generated by the decoction of the mugwort plant. Another good recipe to complement this treatment is to dilute a glass of culantrillo water in a glass of wine and drink three times a day

APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
Mugwort is one of the oldest medicinal plants in the world. According to Greek mythology, the goddess Artemisia, protector of nature, helped women in childbirth; hence the name of this plant that has, among others, properties against menstrual or postpartum pains.
Of potent bitter taste,itis known as Wormwood, bitter mugwort or holy herb.
Mugwort (ARTEMISIA VULGARIS) Cut plant.
Mugwort is one of the oldest medicinal plants in the world
Artemisia is one of the oldest medicinal plants in the world. According to Greek mythology, the goddess Artemisia, protector of nature, helped women in childbirth; hence the name of this plant that has, among others, properties against menstrual pain or postpartum.
It is a medicinal herb whose properties have been known since the time of the pharaohs. Of potent bitter taste because of one of its components, the absintin.
It is a medicinal herb whose properties have been known since the time of the pharaohs
PROPERTIES: Antimicrobial, emmenagogue, astringent, aperient and eupeptic. INDICATIONS:
Anorexia, slow digestion, amenorrhea, dysmenorrhea. PRECAUTIONS:
Do not use it continuously. Do not use it continuously
CONTRAINDICATIONS: CONTRAINDICATIONS: Pregnancy and pregnancy
Pregnancy and lactation. CONTRAINDICATIONS
(SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) (SOURCE: VADEMECUM PLANTAS MEDICINALES PLAMECA) Menstrual disorders : The infusion of the leaves at a rate of 10 g per liter gives benefits as an emmenagogue, so it is recommended in menstrual disorders, such as dysmenorrhea and amenorrhea. At the digestive level : Being a bitter tonic, it is recommended to improve bile secretion, as well as appetite stimulant. At the digestive level : As a bitter tonic, it is recommended to improve bile secretion, as well as appetite stimulant
For diabetes : Prolonged treatments based on mugwort, in a minimum of 3 months, are recommended for cases of diabetes, as it has certain qualities that lower glucose
Organoleptic characteristics: Preservation Keep in a cool, dry place, away from strong odors and sources of contamination. DECLARATION OF ALLERGENS DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED Benefits of Mugwort
In those cases where menstruation does not come and there is no likelihood of pregnancy is very useful to accelerate the process and end the wait by applying vaginal vapors generated by the decoction of the mugwort plant. Another good recipe to complement this treatment is to dilute a glass of culantrillo water in a glass of wine and drink three times a day


APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
