My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
Without gluten

Without gluten
Commonly called pigeonweed or blood of Christ, is a species of annual herbaceous plant of the genus Fumaria in the family Papaveraceae, native to Europe
It possesses emollient, depurative, tonic, hypertensive, hyposthenic, diuretic and anti-inflammatory intestinal properties.
Because of its bitter taste stimulates the digestive substances, being an appetite enhancer
Fumitory plant (FUMARIA OFFICINALIS L.), cut.
Fumitory plant (FUMARIA OFFICINALIS L.), cut
Greyish-green to bluish-green in color; contains numerous bipinnatisect leaf fragments and parts of the hollow, angular stem.
Fumitory plant (FUMARIA OFFICINALIS L.)
Fumitory plant (FUMARIA OFFICINALIS L.)
The crinkled flowers are characteristic, ranging from light purplish to dark purplish and have a dark purple or brownish spot at the apex.
Flowers are characteristic
The fruits are spherical, brownish-green and contain a small brown seed. Somewhat bitter and slightly salty
Commonly called palomilla or sangre de Cristo -among many other names-, it is a species of annual herbaceous plant of the genus Fumaria in the family Papaveraceae, native to Europe.
The fumitory has properties that make it a very popular herbaceous plant
Fumitory has emollient, depurative, tonic, hypertensive, hyposthenic, diuretic and anti-inflammatory intestinal properties.
For the appetite
For the appetite : For its bitter taste stimulates the digestive substances, being an appetite enhancer.
For the liver : For its bitter taste stimulates the digestive substances, being an appetite enhancer.
For the appetite
For the liver : This plant is attributed cholagogue actions, (increases the production of bile secretions, which in turn facilitate the digestive juices the dissolution of fatty acids). For other liver disorders the juice is taken, which is used to control jaundice and hepatitis
Skin problems : This plant has great healing properties at the dermatological level, as the juice can cure and reduce acne, scabies and dermatosis. The plant is crushed in a mortar with a little water, then the resulting mass is pressed, or in a napkin, the mixture is left to stand for a few hours to separate the solid parts and filtered through a strainer or a fine cloth. It is applied on the injured skin.
USE
50 gr. of Fumaria officinalis per liter of water. Leave to infuse 15 minutes and drink 2 to 3 cups per day, before meals (as a tonic, anti-anemic).
Only 10 days per month as prolonged use is soothing, sleepy and slimming. Preservation Keep in a cool, dry place, away from strong odors and sources of contamination
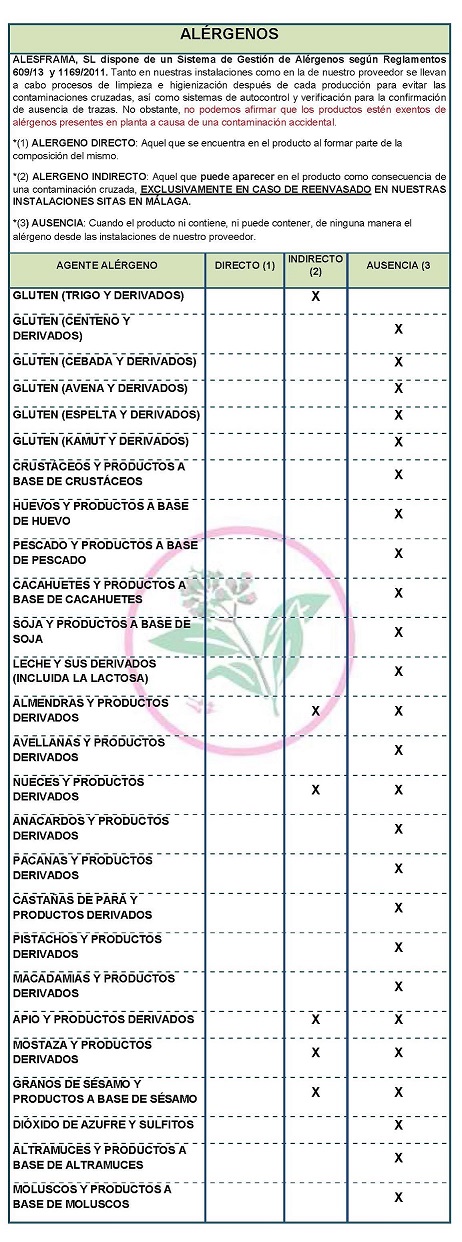
DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS: HALAL FITNESS : YES, official certificate not available DIABETICS SUITABILITY : YES FIT FOR VEGETARIANS : YES FIT FOR VEGANISTS : YES FIT FOR VEGANS : YES CELIACS SUITABILITY : NO NOT SUITABLE FOR CELIACS : NO
Commonly called pigeonweed or blood of Christ, is a species of annual herbaceous plant of the genus Fumaria in the family Papaveraceae, native to Europe
It possesses emollient, depurative, tonic, hypertensive, hyposthenic, diuretic and anti-inflammatory intestinal properties.
Because of its bitter taste stimulates the digestive substances, being an appetite enhancer
Fumitory plant (FUMARIA OFFICINALIS L.), cut.
Fumitory plant (FUMARIA OFFICINALIS L.), cut
Greyish-green to bluish-green in color; contains numerous bipinnatisect leaf fragments and parts of the hollow, angular stem.
Fumitory plant (FUMARIA OFFICINALIS L.)
Fumitory plant (FUMARIA OFFICINALIS L.)
The crinkled flowers are characteristic, ranging from light purplish to dark purplish and have a dark purple or brownish spot at the apex.
Flowers are characteristic
The fruits are spherical, brownish-green and contain a small brown seed. Somewhat bitter and slightly salty
Commonly called palomilla or sangre de Cristo -among many other names-, it is a species of annual herbaceous plant of the genus Fumaria in the family Papaveraceae, native to Europe.
The fumitory has properties that make it a very popular herbaceous plant
Fumitory has emollient, depurative, tonic, hypertensive, hyposthenic, diuretic and anti-inflammatory intestinal properties.
For the appetite
For the appetite : For its bitter taste stimulates the digestive substances, being an appetite enhancer.
For the liver : For its bitter taste stimulates the digestive substances, being an appetite enhancer.
For the appetite
For the liver : This plant is attributed cholagogue actions, (increases the production of bile secretions, which in turn facilitate the digestive juices the dissolution of fatty acids). For other liver disorders the juice is taken, which is used to control jaundice and hepatitis
Skin problems : This plant has great healing properties at the dermatological level, as the juice can cure and reduce acne, scabies and dermatosis. The plant is crushed in a mortar with a little water, then the resulting mass is pressed, or in a napkin, the mixture is left to stand for a few hours to separate the solid parts and filtered through a strainer or a fine cloth. It is applied on the injured skin.
USE
50 gr. of Fumaria officinalis per liter of water. Leave to infuse 15 minutes and drink 2 to 3 cups per day, before meals (as a tonic, anti-anemic).
Only 10 days per month as prolonged use is soothing, sleepy and slimming. Preservation Keep in a cool, dry place, away from strong odors and sources of contamination
DECLARATION OF ALLERGENS According to the data sheet of the supplier(s): Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED. ALLERGENS: Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame. Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS: HALAL FITNESS : YES, official certificate not available DIABETICS SUITABILITY : YES FIT FOR VEGETARIANS : YES FIT FOR VEGANISTS : YES FIT FOR VEGANS : YES CELIACS SUITABILITY : NO NOT SUITABLE FOR CELIACS : NO

