My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !

Without gluten

Vegan

Without gluten

Vegan
Whole juniper berries (JUNIPERUS COMMUNIS)
Whole juniper berries (JUNIPERUS COMMUNIS)
In cooking, juniper berries are ideal for flavoring meat dishes, especially accompanying strong meats such as game. It is used in traditional dishes such as Choucrout, and are included in the preparation of marinades, sauces, etc.
Whole juniper berries (JUNIPERUS COMMUNIS)
Joint juniper berries (JUNIPERUS COMMUNIS)
Juniper is a large shrub
Juniper is a large shrub, 1 or 2 meters high, which if left to grow to its full height can become a tree of several meters. The fruits are made up of berries that when fully ripe take on a dark bluish violet color and become fleshy and consistent. They have a sweet resinous flavor and a certain aroma similar to cinnamon
Gin is the product resulting from the distillation of juniper berries; then each brand adds other botanicals in different proportions, which makes each gin is different. The contribution of these berries enhances and reinforces the original flavor of the gin
In the kitchen, juniper berries are ideal for flavoring meat dishes, especially accompanying strong meats such as game. It is used in traditional dishes such as Choucrout, and are included in the preparation of marinades, sauces, etc.
PROPERTIES OF JUNIPER BERRIES:
Juniper berries have a widely demonstrated antiseptic action, and is one of the most potent herbal solutions for eliminating bacteria from the urinary system.
/p>Juniper berries have a widely demonstrated antiseptic action, and is one of the most potent herbal solutions for eliminating bacteria from the urinary system
USE
To eradicate a beginning urinary tract infection, pour a heaping teaspoon of freshly crushed berries into a cup of boiling water. Cover and let it infuse for 20 minutes. You should drink 2 to 3 cups a day for 5 to 7 days (no longer).
It is only contraindicated in case of renal insufficiency or renal inflammation (nephritis or glomerulonephritis) confirmed by clinical diagnosis.
Juniper berries should be used for short periods of time (which in medicine is considered to range from three to seven days), as their draining effect is so intense that, in the long term, it could lead to irritation of the urinary tract. But you can rest assured that a short administration carries no risk.
Carbohydramine is not recommended for use in the urinary tract
Organoleptic characteristics
Appearance : Correct
Odor : Typical without presence of foreign odors
Color : Black
Preservation
Keep in a cool and dry place, away from strong odors and sources of contamination
Storage
Keep in a cool, dry place, away from strong odors and sources of contamination.

DECLARATION OF ALLERGENS
DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free from allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 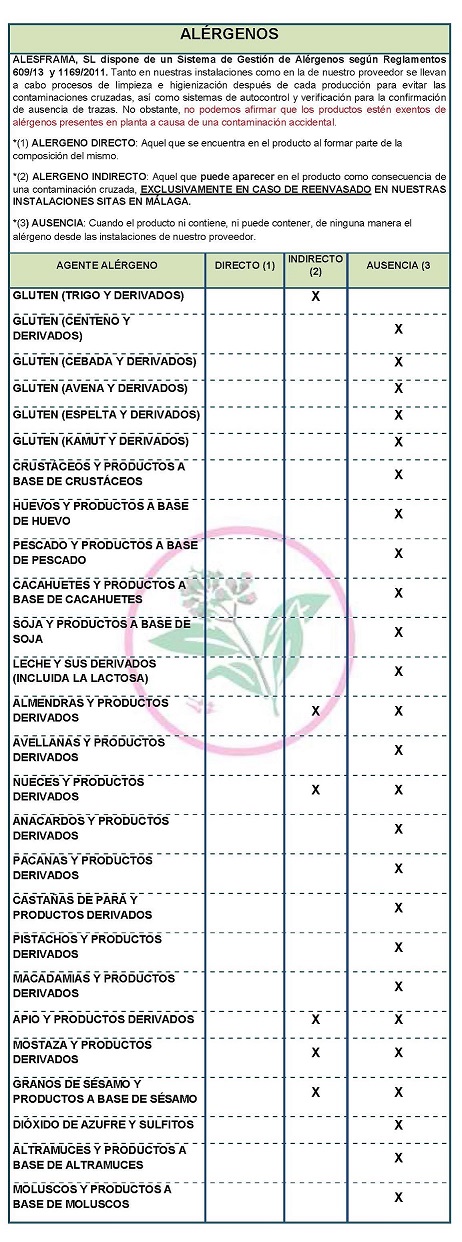
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
Whole juniper berries (JUNIPERUS COMMUNIS)
Whole juniper berries (JUNIPERUS COMMUNIS)
In cooking, juniper berries are ideal for flavoring meat dishes, especially accompanying strong meats such as game. It is used in traditional dishes such as Choucrout, and are included in the preparation of marinades, sauces, etc.
Whole juniper berries (JUNIPERUS COMMUNIS)
Joint juniper berries (JUNIPERUS COMMUNIS)
Juniper is a large shrub
Juniper is a large shrub, 1 or 2 meters high, which if left to grow to its full height can become a tree of several meters. The fruits are made up of berries that when fully ripe take on a dark bluish violet color and become fleshy and consistent. They have a sweet resinous flavor and a certain aroma similar to cinnamon
Gin is the product resulting from the distillation of juniper berries; then each brand adds other botanicals in different proportions, which makes each gin is different. The contribution of these berries enhances and reinforces the original flavor of the gin
In the kitchen, juniper berries are ideal for flavoring meat dishes, especially accompanying strong meats such as game. It is used in traditional dishes such as Choucrout, and are included in the preparation of marinades, sauces, etc.
PROPERTIES OF JUNIPER BERRIES:
Juniper berries have a widely demonstrated antiseptic action, and is one of the most potent herbal solutions for eliminating bacteria from the urinary system.
/p>Juniper berries have a widely demonstrated antiseptic action, and is one of the most potent herbal solutions for eliminating bacteria from the urinary system
USE
To eradicate a beginning urinary tract infection, pour a heaping teaspoon of freshly crushed berries into a cup of boiling water. Cover and let it infuse for 20 minutes. You should drink 2 to 3 cups a day for 5 to 7 days (no longer).
It is only contraindicated in case of renal insufficiency or renal inflammation (nephritis or glomerulonephritis) confirmed by clinical diagnosis.
Juniper berries should be used for short periods of time (which in medicine is considered to range from three to seven days), as their draining effect is so intense that, in the long term, it could lead to irritation of the urinary tract. But you can rest assured that a short administration carries no risk.
Carbohydramine is not recommended for use in the urinary tract
Organoleptic characteristics
Appearance : Correct
Odor : Typical without presence of foreign odors
Color : Black
Preservation
Keep in a cool and dry place, away from strong odors and sources of contamination
Storage
Keep in a cool, dry place, away from strong odors and sources of contamination.

DECLARATION OF ALLERGENS
DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free from allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
