My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart
{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
Without gluten

Without gluten
Hernaria, also known as stonebreaker or stonebreaker, has been used since ancient times by different civilizations to dissolve kidney stones. Traditional Chinese medicine also advises it as hepatoprotective.
Use: In a liter of boiling water add 60 grams of herniaria. Remove from heat and let stand for a minimum of 10 minutes. Strain and sweeten to taste
Hernaria, also known as stonebreaker or chancapiedras has been used since ancient times by different civilizations to dissolve kidney stones. Traditional Chinese medicine also advises it as a hepatoprotective.
Infusion: In a liter of boiling water add 60 grams of herniaria. Remove from heat and let stand for at least 10 minutes. Strain and sweeten to taste, as it has a certain bitter taste in the mouth.
For kidney stones, it is advisable to consume three cups of this infusion per day for 30 days in a row, even if the stones have been expelled.
For kidney stones, it is advisable to consume three cups of this infusion per day for 30 days in a row, even if the stones have been expelled
Same intake for bladder stones.
Origin: Spain ORGANOLEPTIC CHARACTERISTICS CARACTERISTICS
COLOR: Green, brownish green ODOR: Characteristic, without presence of foreign odors APPEARANCE: Correct Appearance
Storage Storage
Keep in a cool, dry place, away from strong odors and sources of contamination Store in a cool, dry place, away from strong odors and sources of contamination
VADEMECUM: (source: https://enestadobeta.com/fitoterapia/web/plantas/279.html)
DECLARATION OF ALLERGENS
According to the technical data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 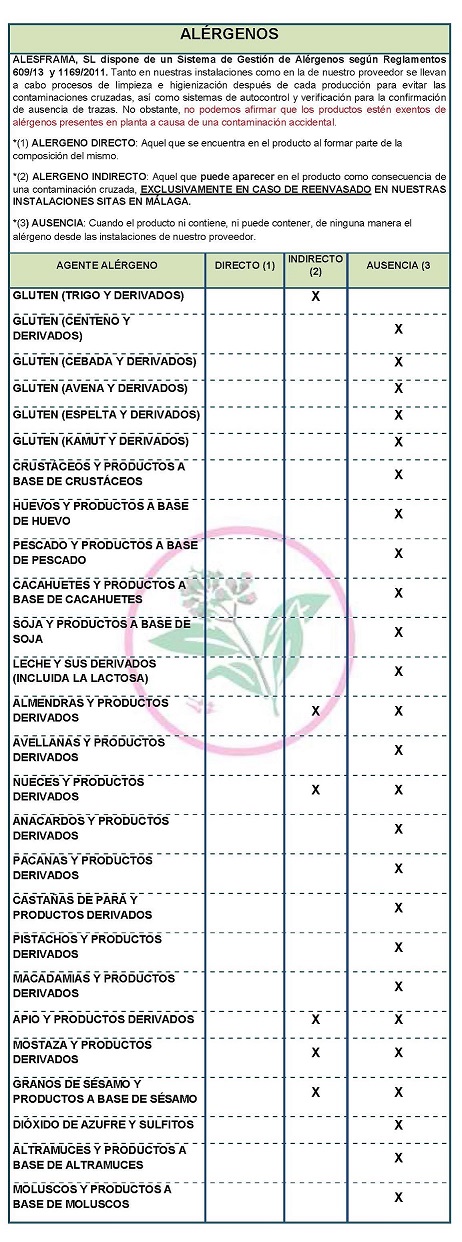
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
Hernaria, also known as stonebreaker or stonebreaker, has been used since ancient times by different civilizations to dissolve kidney stones. Traditional Chinese medicine also advises it as hepatoprotective.
Use: In a liter of boiling water add 60 grams of herniaria. Remove from heat and let stand for a minimum of 10 minutes. Strain and sweeten to taste
Hernaria, also known as stonebreaker or chancapiedras has been used since ancient times by different civilizations to dissolve kidney stones. Traditional Chinese medicine also advises it as a hepatoprotective.
Infusion: In a liter of boiling water add 60 grams of herniaria. Remove from heat and let stand for at least 10 minutes. Strain and sweeten to taste, as it has a certain bitter taste in the mouth.
For kidney stones, it is advisable to consume three cups of this infusion per day for 30 days in a row, even if the stones have been expelled.
For kidney stones, it is advisable to consume three cups of this infusion per day for 30 days in a row, even if the stones have been expelled
Same intake for bladder stones.
Origin: Spain ORGANOLEPTIC CHARACTERISTICS CARACTERISTICS
COLOR: Green, brownish green ODOR: Characteristic, without presence of foreign odors APPEARANCE: Correct Appearance
Storage Storage
Keep in a cool, dry place, away from strong odors and sources of contamination Store in a cool, dry place, away from strong odors and sources of contamination
VADEMECUM: (source: https://enestadobeta.com/fitoterapia/web/plantas/279.html)
DECLARATION OF ALLERGENS
According to the technical data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
At both our facilities and our supplier's, cleaning and sanitizing processes are carried out after each production to avoid cross-contamination, as well as self-monitoring and verification systems for the confirmation of absence of traces,. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
