My baskets
You still don't have anything in your shopping cart
My baskets
You still don't have anything in your shopping cart

{{getOldPrice()}}{{getPrice()}}
 ¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
¡ Buying this product you get {{calculatedProductMenttos()}} menttos !
Without gluten

Without gluten
Because of its composition and active ingredients, it has antihemorrhagic, healing, antifungal, diuretic, astringent and remineralizing action
Excellent diuretic, indicated in renal disorders and inflammation of the urinary bladder and prostate, and against fluid retention. contributes to purify the blood and ultimately reduces certain skin conditions
Horsetail (EQUISETUM sps L) cut plant
By its composition and active ingredients (saponosides, tannins, flavonoids, alkaloids and mineral salts, especially silica and potassium, and vitamin C) has antihemorrhagic, healing, antifungal, diuretic, astringent and remineralizing action
The great secret of horsetail is its silica content, a substance associated with growth processes, which is present in the lungs, brain, liver and muscles, as well as in nails, hair, skin and connective tissue. Thanks to its silica and potassium content, it is an excellent diuretic, indicated for kidney disorders and inflammations of the urinary bladder and prostate, and for those who need to increase the amount of urine due to a tendency to retain liquids. By cleansing the urinary tract (detoxifying action), it helps to purify the blood and ultimately reduces certain skin conditions. It is therefore very indicated in case of eczema and herpes, as well as skin fungus
Indications: post-traumatic edema, to increase diuresis ("flushing therapy") in infectious or inflammatory conditions of the urinary tract (oral route) and as an aid in the healing of wounds and torpid ulcers in topical use.
It has been traditionally used to increase diuresis ("flushing therapy") in infectious or inflammatory conditions of the urinary tract (oral route) and as an aid in the healing of wounds and torpid ulcers in topical use
Traditionally it has been used in the form of herbal teas to increase diuresis, often as an adjuvant in the treatment of overweight.
Traditionally it has been used in the form of herbal teas to increase diuresis, often as an adjuvant in the treatment of overweight
USES The most common way to take it is in infusion, boiling the plant for five minutes and letting it stand for another five. If we want to treat a particular ailment, we recommend taking three glasses daily for a month and a half, and then rest for a few weeks
The treatment can be repeated throughout the year. The treatment can be repeated throughout the year
Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. Consistency
Storage
Keep in a cool, dry place, away from strong odors and sources of contamination.

DECLARATION OF ALLERGENS
DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 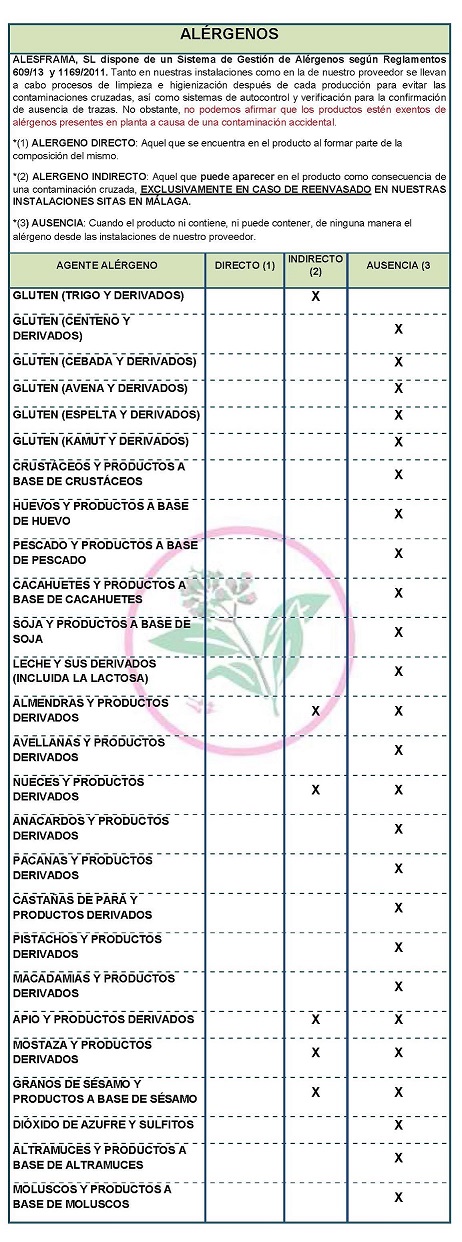
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
Because of its composition and active ingredients, it has antihemorrhagic, healing, antifungal, diuretic, astringent and remineralizing action
Excellent diuretic, indicated in renal disorders and inflammation of the urinary bladder and prostate, and against fluid retention. contributes to purify the blood and ultimately reduces certain skin conditions
Horsetail (EQUISETUM sps L) cut plant
By its composition and active ingredients (saponosides, tannins, flavonoids, alkaloids and mineral salts, especially silica and potassium, and vitamin C) has antihemorrhagic, healing, antifungal, diuretic, astringent and remineralizing action
The great secret of horsetail is its silica content, a substance associated with growth processes, which is present in the lungs, brain, liver and muscles, as well as in nails, hair, skin and connective tissue. Thanks to its silica and potassium content, it is an excellent diuretic, indicated for kidney disorders and inflammations of the urinary bladder and prostate, and for those who need to increase the amount of urine due to a tendency to retain liquids. By cleansing the urinary tract (detoxifying action), it helps to purify the blood and ultimately reduces certain skin conditions. It is therefore very indicated in case of eczema and herpes, as well as skin fungus
Indications: post-traumatic edema, to increase diuresis ("flushing therapy") in infectious or inflammatory conditions of the urinary tract (oral route) and as an aid in the healing of wounds and torpid ulcers in topical use.
It has been traditionally used to increase diuresis ("flushing therapy") in infectious or inflammatory conditions of the urinary tract (oral route) and as an aid in the healing of wounds and torpid ulcers in topical use
Traditionally it has been used in the form of herbal teas to increase diuresis, often as an adjuvant in the treatment of overweight.
Traditionally it has been used in the form of herbal teas to increase diuresis, often as an adjuvant in the treatment of overweight
USES The most common way to take it is in infusion, boiling the plant for five minutes and letting it stand for another five. If we want to treat a particular ailment, we recommend taking three glasses daily for a month and a half, and then rest for a few weeks
The treatment can be repeated throughout the year. The treatment can be repeated throughout the year
Conservation Keep in a cool, dry place, away from strong odors and sources of contamination. Consistency
Storage
Keep in a cool, dry place, away from strong odors and sources of contamination.

DECLARATION OF ALLERGENS
DECLARATION OF ALLERGENS
According to the data sheet of the supplier(s):
Contains NO GLUTEN AS AN INGREDIENT, BUT THE ABSENCE OF TRACE CANNOT BE GUARANTEED.
ALLERGENS:
Contains no allergens as ingredients
Because of cross-contamination may contain Celeriac, nuts, mustard and sesame.
Cleaning and sanitizing processes are carried out both in our facilities and in our supplier's facilities after each production to avoid cross-contamination, as well as self-control and verification systems to confirm the absence of traces. However, we cannot claim that the products are free of allergens present in the plant due to accidental contamination
COMMUNITY DIRECTIVE 2006/142/EC ON INGREDIENTS AND LABELLING OF ALLERGENS According to directive 2003/89/EC below is a list of allergenic ingredients that must be mentioned in the labeling for the purpose of consumer protection since the presence of these substances can cause allergies. DECLARATION OF NOT GENETICALLY MODIFIED 
APPLICABLE LEGISLATION
APTITUDE FOR ETHNIC GROUPS AND OTHERS:
HALAL FITNESS : YES, official certificate not available
DIABETICS SUITABILITY : YES
FIT FOR VEGETARIANS : YES
FIT FOR VEGANISTS : YES
FIT FOR VEGANS : YES
CELIACS SUITABILITY : NO
NOT SUITABLE FOR CELIACS : NO
